BreatheSuite / coverage
coverage
coverage summary


About
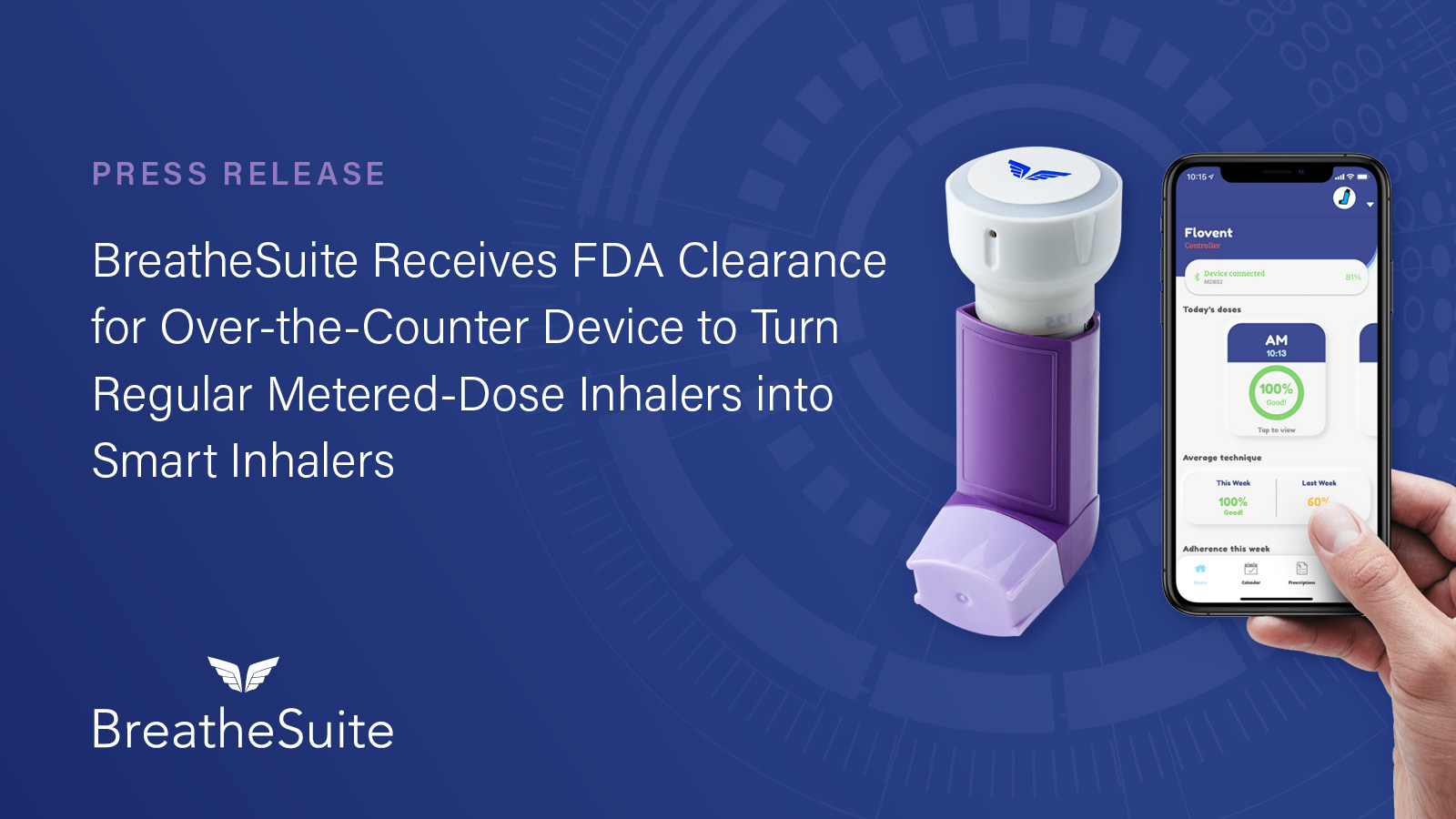
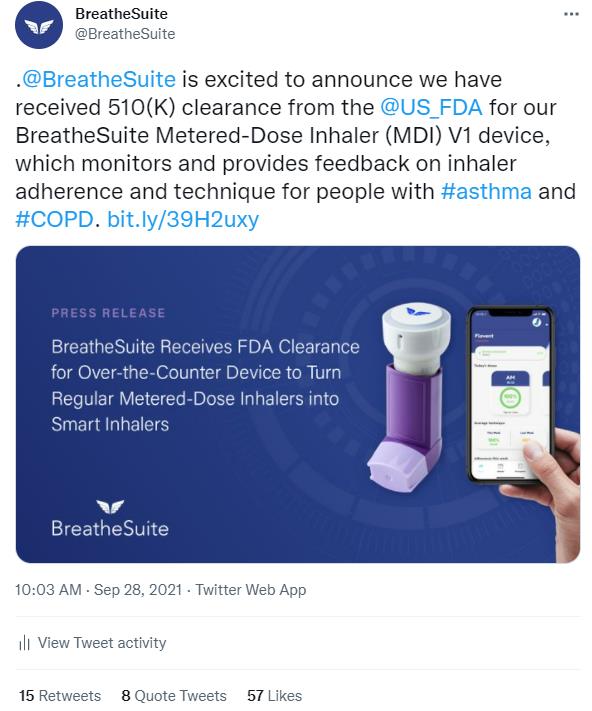
In September 2021, BreatheSuite announced that their connected health device for chronic respiratory conditions had been cleared by the US Food and Drug Administration. As a result of our press release, social media push and targeted media outreach, SVM secured coverage with FDA News, HIT Consultant, mHealth Times, MassDevice and RT Magazine. SVM promoted the news release and resulting media coverage with social media posts on BreatheSuite’s Twitter and LinkedIn accounts.